Validation & Quality Assurance
Quality Assurance Consulting
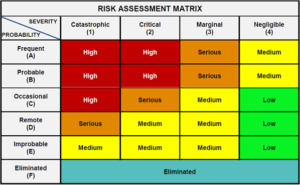
Change Control, ASTM E2500-07, Risk assessment, ICH Guidelines, CMC consulting, validation planning and execution: we can help your life sciences group with QA challenges.
Innovative consultants understand and carefully follow SDLC best practices, or those best practices adopted by our clients. We have experience with a wide range or manufacturing, lab, and enterprise hardware and software systems. Let us help you with your CSV needs.
Computer Systems Validation


Cleaning
Validation
With extensive experience in processes such as Clean-in Place (CIP), Steam-in-Place (SIP) and smoke studies, let us help to ensure sanitary, compliant and efficient operations in your regulated environment.
We supply validation and quality professionals to leading biotech and pharmaceutical companies for extended project and operations needs.
Validation Staff Augmentation

307 Waverley Oaks Rd.
Waltham, MA 02452
(978) 369-9020
© 2024 All Rights Reserved | Innovative Process Solutions, Inc. | Privacy Policy

